
Loftware の業界をリードするラベル管理ソリューションで、進化する FDA の規制に先んじて対応しましょう。食品・飲料、医薬品、医療機器業界のメーカーとブランド向けに設計された当社のクラウドベース プラットフォームは、正確性、制御性、速度を提供し、コンプライアンスの確保、リスクの軽減、消費者の保護を、プロセス全体を通じて実現します。
米国食品医薬品局(FDA)は、連邦政府の機関として、食品、飲料、医薬品、医療機器、その他の消費財の安全性、有効性、適切なラベル表示を確保し、公衆の健康を保護する責任を負っています。
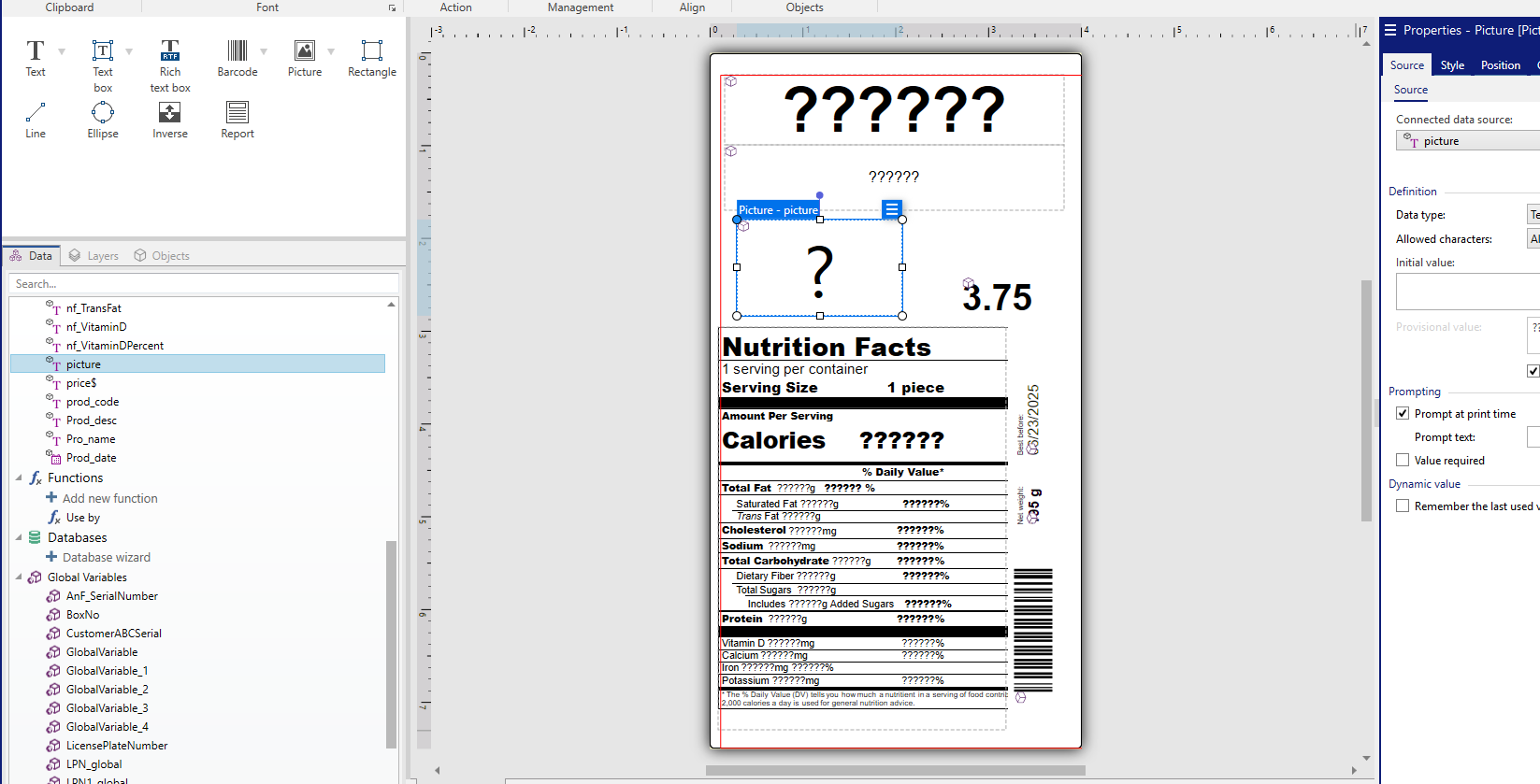
規制対象業界の企業に対し、FDA は製品ラベルに表記しなければいけない内容に関する厳格なガイドラインを設定しています。これには栄養情報、アレルゲン表示、原材料一覧、健康に関する表示などが含まれます。これらのラベル表示基準は、透明性を促進し、消費者への危害を防止し、情報に基づいた購入判断を支援することを目的としています。


Loftware は、ラベル管理ソリューションを通じて、進化する FDA 規制に先んじて対応し、コンプライアンスを確実にするだけなく、リスクを軽減し、グローバルなサプライチェーンの業務を効率化します。

ラベル管理システムを直接 ERP、MES、PLM プラットフォームと統合することで、精度を最適化し、エラーを削減します。FDA が定める必須項目(バッチ番号、栄養成分表示、アレルゲン表示、原材料一覧など)を自動的に入力し、すべてのラベルが最新かつ検証済みのデータと一致するようにします。
Loftware のルールベースエンジンを活用し、容器包装前面表示(FoP)による栄養表示など、変化する規制要件に柔軟に対応でき、ラベルのサイズ、レイアウト、コンテンツを自動的に調整できます。FDA がデータの一貫性とアクセス性を向上させるため、構造化され標準化されたフォーマットを推進する中、Loftware は数多くの静的ファイルを管理することなく、ラベル管理を簡素化できます。
高額なリコールを回避し、米国および国際的な規制(CGMP、CFR 21、FSMA、UDI、GHS、FALCPA、EU 1169、FDA のトレーサビリティ規則、SPL 電子ラベル、ナターシャ法、FOP など)へのエンドツーエンドの準拠を確保します。Loftware は、ビジネス規模に合わせて拡張可能な単一のセキュアなプラットフォームから、すべてのコンプライアンス活動を管理できます。
企業全体でのラベル管理活動の可視化を実現し、監査の迅速化、予防的なリコール、および偽造防止対策を支援します。Loftware の一元化された制御フレームワークは、製造から流通までのトレーサビリティを確保し、FDA の記録管理および報告要件に不可欠な機能を提供します。
FDA の「健康」に関する新たな定義など、規制変更に IT 部門の負担を増やすことなく対応できます。ビジネスユーザーは、汎用テンプレートを簡単に活用してラベルの変更を適用し、請求内容を検証し、コンプライアンスを維持できます。これにより、市場投入までの時間を短縮し、手作業によるエラーのリスクを軽減します。
アレルゲン表示ガイドラインの改訂から植物由来製品に関する新たな表明まで、今後の変化に備えましょう。Loftware の柔軟に設定可能なクラウドベースのラベル管理ソリューションは、FDA の現行および将来の規制への迅速な準拠をサポートし、長期的な柔軟性と安心を提供します。
Loftware のクラウドベースのラベル管理ソリューションを活用することで、当社は FDA 規制への準拠を確保し、市場投入プロセスを加速することができました。
Michael R. Kinnett
医療製品部門、製品のラベル管理
当社を導入し、SAP と統合して以来、私たちは製造施設全体でラベル管理を拡大することができ、同時に新しいお客様の要望や追加の規制要件にうまく対応することができようになりました。
Tim Cukr
IT プロフェッショナル
Loftware は、当社のラベルの納期を70%短縮し、FDA のコンプライアンス要件を効率的かつ正確に満たすことを可能にしました。

Randy Cochran
プロジェクトマネージャー
当社は現在、医療機器のグローバル市場へのアクセスを確実にし、必要に応じてラベルの内容をカスタマイズすることができるようになったため、製品承認の迅速化と規制遅延の削減につながっています。
Mark Illgner
ラベル開発シニアエンジニア
品質と生産の面において、当社はすべてのデータを一元管理し、現地で展開しています。これにより、常に正しいデータが印刷され、規制遵守が確保されています。

Lucy Webb
テクニカル マネージャー
